Bacteriophages were first used therapeutically in humans in 1919 (shortly after their discovery), to treat severe cases of bacterial dysentery in four children in Paris, France. All of the treated children recovered from what otherwise could have been a fatal infection. The study was conducted in close collaboration with Felix d'Herelle, the discoverer of bacteriophages. However, it was not published in the scientific literature until several years later1. Therefore, the first published report about using bacteriophages to treat human infections dates to 1921, when Richard Bruynoghe and Joseph Maisin published a short paper describing the successful use of bacteriophages to treat staphylococcal skin disease in six patients2. Since that time, phages have been used to treat bacterial infections of humans in a variety of clinical settings. Phages have been administered to humans:
- Orally, in tablet and liquid formulations
- Rectally
- Locally (skin, eye, ear, nasal mucosa, etc.), in tampons, rinses and creams
- As aerosols and intrapleural injections
- Intravenously
There have been no reports of serious complications associated with the use of phages in such settings 1,3.
Therapeutic phage preparations also were produced in the United States during the 1930s and 40s, by well-known pharmaceutical companies, including Eli Lilly, E. R. Squibb and Sons, and Swan-Myers (a division of Abbott Laboratories). However, the advent of antibiotics caused interest in phage therapy to decline in the West, and therapeutic phage applications were all but abandoned in the United States, Western Europe and the rest of the developed world.
At the same time, therapeutic phage applications continued in the former Soviet Union (FSU) and many Eastern European countries, where therapeutic phage preparations are currently available for sale in pharmacies and specialized research centers and clinics. See Sulakvelidze and Kutter4 for a more in-depth review of phage therapy in humans.
Emergence Of Antibiotic Resistance and Its Implications
The rapid and alarming emergence of antibiotic-resistant "superbugs" has rekindled interest in phage therapy in the West. Indeed, the increasing emergence of antibiotic-resistant bacterial pathogens may have very dangerous public health ramifications, and it may seriously impact the way medicine is practiced today in much of the world. The problem was emphasized in a report by a special Task Force co-chaired by the CDC, FDA and NIH, which stated that "the world may soon be faced with previously treatable diseases that have again become untreatable, as in the pre-antibiotic era."
Phage therapy can be a potent tool for dealing with bacterial infections of humans, and that phage therapy will help to reduce problems caused by the emergence of antibiotic-resistant bacteria (i.e., bacteria that are untreatable with currently available antibiotics). In many cases, it may be the only safe and effective approach currently available for saving a patient's life.
The Impact Of The Microbiome On Human Health
The human microbiome comprises hundreds of bacterial species and is an important factor in determining an individual's health. Diversity or the balance of "good" and "bad" bacteria in the gut microbiome helps maintain health through multiple mechanisms, including modulation of inflammation and regulation of protective gastrointestinal functions. While the overall features of the gut microbiome appear to be stable in adults, diet has been shown to alter the composition of both good and bad bacteria in the gut lending support to the idea that probiotics and dietary supplements can be used as modulators of the microbiome. In this context, lytic bacteriophages are superbly suited for gentle and targeted fine-tuning of the microbiome by killing their specific targeted bacterial pathogens without disturbing the normal microflora - a unique biological property that is increasingly explored for developing novel tools for microbiome modulation and research.
Bacteriophages provide a technology platform to support healthy gut microbiomes and prevent and/or treat acute bacterial infections. The key advantages to phage therapy are that:
- the non-antibiotic-based mechanisms of action prevent the development of antibiotic resistance;
- it allows for targeting of multi-antibiotic-resistant bacteria that are otherwise refractory to the current standard of care; and,
- depending on its application, it could reduce the incidence of post-infectious sequelae associated acute enteric infections, such as inflammatory bowel diseases and reactive arthritis.
Moreover, this technology could be applicable to other acute and antibiotic resistant bacterial infections and lead to improved prevention, treatment and quality of life.
Despite widespread use of bacteriophage products in humans in Eastern Europe and the Soviet Union, bacteriophages still are not approved for use in the U.S. or European Union for clinical applications. Intralytix has led the way in creating a road map for FDA regulatory approvals in the U.S. from food safety applications to investigational new drugs for human therapy. In 2005, Intralytix filed the first and only Drug Master File for a bacteriophage product. Under the first FDA approved Investigation New Drug (IND) application for a bacteriophage product, we completed the first U.S. clinical trial of a topically applied bacteriophage product in 20085. Intralytix continues to pave new pathways for human bacteriophage-based products with the 2018 IND FDA approval of an oral bacteriophage treatment in the first attempt to use bacteriophage therapy to manage Crohn's disease in humans.
Intralytix currently has a number of human phage therapy products in its pipeline to address various urgent and unmet medical needs. Products under various stages of development include bacteriophages to target:
- Adherent-invasive E. coli associated with Crohn's disease (EcoActive™)
- Shigellosis (ShigActive™)
- Women's health associated bacterial infections, including bacterial vaginosis and pregnancy-associated infections
- Antibiotic resistant Enterococcus colonization and associated bacteremia (VRELysin™)
Clinical Trials
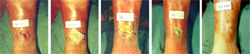
One of Intralytix's prototype phage preparations for treating infected wounds was successfully used during the Phase 1 human clinical trial in Lubbock, Texas5. This product is not yet available for clinical applications.
Intralytix's EcoActive™ bacteriophage therapy targeting adherent-invasive E. coli (AIEC) in Crohn's disease patients entered a Phase 1/2a clinical trial in 2019 and is currently enrolling participants at the Icahn School of Medicine at the Mount Sinai Hospital in New York, NY. The trial, registered under clinicaltrials.gov NCT03808103, is currently recruiting volunteers with inactive Crohn's disease. For questions on phage study eligibility, please contact the study coordinator Amy Nolan at amy.nolan@mssm.edu or +1-212-824-7699.
Intralytix has strategic plans to develop several additional products for human therapeutics. Currently we do not produce any FDA approved phage-based commercial preparations for human therapy applications. For further information on new therapies in clinical trials (including phage therapy trials) from various organizations, please visit www.clinicaltrials.gov.
Intralytix has several issued and pending patents pertinent to the use of bacteriophages in microbiome modification and for using phages to eliminate or significantly reduce bacterial colonization in humans and other animals, including US patents #7,459,272 and #8,003,323. These broad patents give Intralytix a significant competitive advantage over other bacteriophage companies, particularly for all "microbiome" applications when phages are used to modify the microbiome of humans by specifically targeting selected bacterial pathogens.
- Sulakvelidze, A., Alavidze, Z., and Morris, J. G., Jr., Bacteriophage therapy, Antimicrob Agents Chemother 45 (3), 649-659, 2001.
- Bruynoghe, R. and Maisin, J., Essais de thérapeutique au moyen du bactériophage du Staphylocoque, J Compt Rend Soc Biol 85, 1120-1121, 1921.
- Alisky, J., Iczkowski, K., Rapoport, A., and Troitsky, N., Bacteriophages show promise as antimicrobial agents, J Infect 36 (1), 5-15, 1998.
- Sulakvelidze, A. and E. Kutter, Bacteriophage therapy in humans, in Bacteriophages: Biology and Application, E. Kutter and A. Sulakvelidze, Editors. 2005, CRC Press: Boca Raton, FL. p. 381-436.
- Wolcott, R., Rhoads, D., Kuskowski, M., Ward, L., and Sulakvelidze, A., Bacteriophage therapy of venous leg ulcers in humans: results of a Phase I safety trial, Journal of Wound Care 18 (6), 237-243, 2009.