Infectious Arms Race Forging new weapons against resurgent bacterial killers
BY THOMAS HAYDEN
Think your job's frustrating? Try a tour of duty in the war against bacteria. Stephen Sanche, an infectious disease specialist in Saskatoon, Canada, has been on the front lines for 12 years now, but the work somehow keeps getting tougher. Take one patient, a 78-year-old retiree with a spinal infection. After two weeks on multiple antibiotics, he was in worse shape than ever. It took 10 weeks and three more powerful antibiotics for him to improve. "The first antibiotics should have been effective," Sanche says. "But every year we're seeing more bacteria more resistant to treatments that worked well when I started."
More than seven decades after a microbe first succumbed to penicillium mold in Alexander Fleming's petri dish, we weren't supposed to be worrying about bacteria anymore. But the bugs are backwith a vengeance. Hospitals, nursing homes, and day-care centers have become the haunts of deadly pathogens, many of them newly impervious to standard treatments. In this, the age of high-tech medicine, the pneumococcus bacterium alone kills some 40,000 Americans every year. And out in the streets, drug-resistant strains of old enemies like tuberculosis and gonorrhea are staging a major resurgence. In his 1992 book The Antibiotic Paradox, Tufts University microbiologist Stuart Levy warned of a looming antibiotic resistance crisis. A decade later, Levy says, it is so much worse he's had to write a new edition. "I don't think calling [antibiotic resistance] a national security issue is overstating it. The bottom line is, we need new drugs."
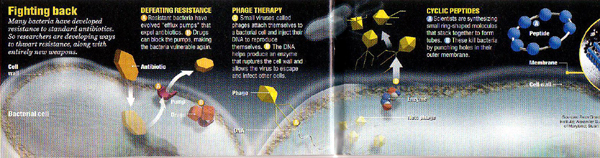
Scientists are answering that call, turning to advances in genomics, molecular biology, and chemical synthesis to develop the first new antibiotics in a generation. First they worked out the genetic tricks that bacteria use to outsmart drugsenzymes to chop antibiotics into harmless pieces, molecular pumps to squirt invading chemicals right back out again. Researchers are now fighting back with tricks of their own, including chemicals that gum up the protective enzymes and molecular corks to block bacterial pumps.
Zappers. Drug developers are also combing bacterial genomestheir genetic parts listsfor new molecular soft spots. Chemists are sorting through hundreds of thousands of chemical compounds, looking for potential bug zappers. In April 2000, Zyvoxapproved for major hospital-acquired infectionsbecame the first entirely new addition to the antibiotic arsenal in 35 years. Another half-dozen drugs are in clinical trials, and labs everywhere are finding novel ways to attack bacteria.
Of all the traditional antibiotics, only the fluoroquinolones (think Cipro) are artificial compounds. We've co-opted the rest from microbe-killing compounds found in nature, where bacteria have had eons to develop ways of protecting themselves. So chemists are now engineering their own molecules, hoping that bacteria will be stymied by unfamiliar toxins.
At the Scripps Research Institute in La Jolla, Calif., Reza Ghadiri is creating doughnut-shaped chemicals called cyclic peptides. The molecules insert themselves into bacterial cell membranes, causing fatal leaks. Compared with traditional antibiotics, the peptides should be harder for bacteria to shrug off, not only because they are fresh weapons but also because they attack a complex structure rather than a single enzyme, Ghadiri says. His team already has successfully cured mice of staph infections with these chemicals. "It's working faster and better than we dreamed," he says. Next step: handing the research off to a drug development company.
Remember Jonathan Swift's beleaguered fleas? "A flea / Has smaller fleas that on him prey; / And these have smaller still to bite 'em; / And so proceed ad infinitum." Bacteria, too, have their pests: tiny viruses called phages that can be wonderfully adept at invading bacteria and bursting them from within. Early attempts to fight bacteria with their viral fleas showed promise, but when penicillin became widely available in the early 1940s, "phage therapy" faded in the West. (Soviet scientists stuck with it, and even now one can buy phage over the counter in the Republic of Georgia.) At the University of Maryland, epidemiologist Alexander Sulakvelidze is taking a second look at the bacteria killers. "We're trying to bring the old technology to a state-of-the- art biotech level," says Sulakvelidze, who once worked at the Georgian institute that did Soviet phage research. With the biotech company Intralytix, Sulakvelidze hopes to start human trials soon.
If you don't relish the thought of swallowing a dose of virusor of old Soviet scienceyou may prefer Vincent Fischetti's approach. The Rockefeller University microbiologist has isolated the enzyme that phages use to chew through bacterial cell walls and, in a study published last week in the journal Science, has shown it can obliterate pneumococcus in mice. Fischetti foresees nasal sprays based on the enzyme that could deal a pre-emptive blow to dangerous germs that lurk in the nose and throat, waiting to cause, among other things, some 10 million childhood ear infections a year. Trials could start next year, he says. "The enzymes are ready to go."
Heeding his own call for new drugs, Levy also aims to stop infection before it starts, by zeroing in on bacterial genetics. A single regulator protein controls 75 to 80 different genes that enable bacteria like salmonella, shi-gella, and E. coli to release toxins and fight antibiotics. Because the bugs often infect surgery patients, notes Levy, a regulatory inhibitor could be given to disarm any invaders before the first cut. "We can't always keep bacteria out of the body," says Levy, "but we may be able to keep them from causing any harm." Several promising inhibitors have been identified, he says, but clinical tests are still years away.
No matter how many new drugs are brought to the battle, evolutionary biologists warn that we'll never win the war with bacteria outright. Because bacteria adapt so readily, says Paul Ewald of Amherst College, "no matter what chemicals we throw at them, they'll find a way around it" in the end.
Life cycles. Yet evolutionary thinking also points to ways of staving off defeat. One is resisting the temptation to prescribe antibiotics "just in case"witness the recent run on Cipro. Another, says Stephen Palumbi of Harvard University, is taking a lesson from farmers, who are fighting the parallel problem of pesticide-resistant insects. Just as farmers are learning to alternate pesticides so the quarry never gets too comfortable with any particular one, doctors might rotate antibiotics as they treat infections. Ewald also suggests paying more attention to the life cycles of disease bacteria. Syphilis, for example, is infectious only for the first few months but causes most of its damage later on. So doctors could reserve their most precious antibioticsthose that remain potentfor later stages, when resistant bacteria can't spread.
But ultimately, says Ewald, we'll have to learn to live with bacteria. Call it a truce, rather than a victory. "Our goal," he says, "should not be to eradicate pathogens but to favor mild strains." Cholera in South America shows how it can happen. In countries with substandard water treatment, cholera remains a killer. But in Chile, with its modern water treatment facilities, the bug has become mildalmost domesticated. The reason? Unable to spread through contaminated water, bacteria that made people too sick to get out of bed never got a chance to infect anyone else. Only milder strains that could spread as their relatively healthy hosts went about their business could flourish.
By changing our own behavior, Ewald says, we can in essence convince some of our most virulent pathogens to change theirs. He's not talking about 21st-century science; strategies as simple as staying at homeand away from the kidswhen you feel too sick to work can help coax pathogenic species to evolve into kinder, gentler versions of their former selves. "Up until now," says Ewald, "evolution has been part of the problem. But it can also be part of the solution."